Interview: Stanley Miller

Biology and chemistry are allied sciences. Nowhere is their interdependence more evident than in the study of the origin of life. Chemists and biologists have long speculated about the conditions of primitive Earth and how that environment could have given rise to the precursors of living cells.
In 1953, when Stanley Miller was only 23 years old, he performed an experiment that would attract global attention. In a laboratory at the University of Chicago, Dr. Miller attempted to simulate the chemical dynamics of the primordial Earth in a glass apparatus that he had constructed. His landmark experiment was the first to test the hypothesis that, given the right conditions, the chemical building blocks of life could originate from simpler chemicals. In this interview, Dr. Miller shares with us the events that led to his experiment and the results that brought the world a step closer to understanding how life on earth began.
Today, Dr. Miller is a professor of chemistry at the University of California, San Diego, where he continues his research on the prebiotic synthesis of organic compounds. In addition to doing research, Dr. Miller teaches courses in biochemical evolution, molecular biochemistry, and physical chemistry.
Thinking back to your student days, can you recall how you chose your schools—Berkeley for your undergraduate education and the University of Chicago for graduate school?
I chose Berkeley because it was logical—right near my home in Oakland—and it was a good school. It is also costly to go away from home. As for graduate school, the chemistry department at Berkeley didn't allow undergraduates to continue on as graduate students. This is common in chemistry departments. The feeling is that chemistry is so diverse that students should broaden their perspective by attending a different school. I talked to a number of professors about which was the best place to go, and at the time, Chicago seemed the best choice. The real problem was whether or not the stipend that I was offered was adequate. Although shopping around for the biggest fellowship is not a good thing to do, you've got to have enough money to live on.
As a chemical system, what most distinguishes life from nonliving matter?
The essential difference between life and nonlife is replication. There are other differences, but this is the essential one. In addition to replication, there has to be mutation, with the mutations transmitted to the progeny. Thus the origin of life is the origin of replication with mutation. Another way to state this is that the origin of life is the origin of evolution, since reproduction or replication, mutation, and selection result in Darwinian evolution.
You're best known for your experiments demonstrating that organic molecules could have been produced on the early Earth before there was life. What is an organic molecule?
The old definition, 100 years ago, was a molecule that came from living organisms. It was felt that carbon compounds, except carbon dioxide and carbon monoxide, came only from living organisms and had some sort of vital force in them. But we now know that there is no vital force and organic compounds are just those that contain carbon.
What experiments were historically important in dispelling the myth that organic molecules are products of supernatural vital forces?
The example that's usually cited is Wohler's synthesis of urea in 1828, in which he took ammonium cyanate and converted it to urea, which is a product of animal excretion. But the idea didn't catch on until people started making acetic acid and other organic compounds from starting materials derived from nonliving components.
Prior to your experiments in the early 1950s, what was the prevalent view among biologists about the origin of the first organic molecules?
As far as I can tell, they really didn't think much about it. There was just no rational explanation. What was usually proposed was that life arose by an extremely improbable event. There was also Oparin's hypothesis, which had been around since 1938. Oparin proposed there was an abundant organic soup, and that the first organisms were derived from these prebiotic organic compounds and ate them rather than making their own.
If organisms didn't make the organic substances present in this primordial soup, then what was the source of these molecules?
Oparin proposed that the primitive atmosphere contained the gases methane, ammonia, and hydrogen, and water and that chemical reactions in that primitive atmosphere produced the first organic molecules. That hypothesis had a good deal of appeal, but without the experiments it was talked about but not very well accepted.

What did you propose to do as an experiment to test the hypothesis?
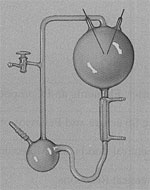
Harold Urey, my advisor at the University of Chicago, knew that it was more reasonable to make organic compounds from the reduced gases in the proposed primitive atmosphere than in our modern atmosphere. But after some discussion, it was felt that if methane, ammonia, and water were combined and just left to sit, organic compounds would not be made. Energy must be added. And for the source of energy, the choices would be ultraviolet light or electrical discharge (a spark). UV light is difficult to work with, so we settled on an electrical discharge, which was handy around most chemical labs in the form of a vacuum leak detector. The plan was to simulate the primitive atmosphere in a glass apparatus, energize the mixture of gases with an electrical spark, and see if I made any organic molecules.
When you first proposed the experiment, what was Dr. Urey's initial reaction?
Well, I went up to Urey and told him that I wanted to do this experiment testing his ideas about the primitive atmosphere and the synthesis of organic compounds. He tried to talk me out of it and get me to do another project. When he realized I was determined, he explained that he felt this was very risky and he was responsible for making certain that I could come up with a Ph.D. thesis in two or three years. So we agreed that we'd give it six months or a year, and if nothing came out of it, we would go back to something conventional. But since we obtained encouraging results quickly, there was never any question that we would continue with it.
When Dr. Urey described your proposed experiment at a seminar, a doubtful listener asked, "And what do you expect to get?" Urey replied "Beilstein." What did he mean?
He was referring to Beilstein's Handbuch der Organischen Chemie. It used to be a single volume, but by now it has grown to about four hundred volumes. These volumes contain a list of properties of every organic compound that's ever been reported, a few million by this time. And what he meant was that we would get every possible type of organic compound formed more or less randomly.
When you actually cranked up your apparatus to simulate early Earth conditions, what did you make?
The surprise was that we didn't get Beilstein; we got mainly organic compounds of biological significance. And the amino acids were formed, not in trace quantities, but abundantly! The experiment went beyond our wildest hopes.
What are amino acids?
Amino acids are the building blocks of proteins. Twenty of them occur in proteins, and many thousands more are possible. Since proteins are important to living organisms, making amino acids would be considered very important.
What was the physical appearance of the soup that you made in your discharge apparatus?
The first time I did the experiment, it turned red. Very dramatic! And then after it turned red, it got more yellow and then brown as the sparking went on. The experiment is easily reproducible with the exception of this red color.
After you'd been on your project for about 3 1/2 months, you were ready to publish your first paper reporting your results. Graduate students usually list their advisors as coauthors on their manuscripts, but when you took your paper to Dr. Urey, he suggested that you remove his name from the manuscript. Why was that?
Well, he felt that if his name had been on the paper he would have gotten all the credit and I would have gotten none. So he took his name off. And even at that, it's known as the Miller-Urey experiment. And it's probably the thing he's best known for, with the exception of the discovery of deuterium, for which he won the Nobel Prize, and his work on the atomic bomb.
After the first paper was published in Science, Urey expressed concern that the amino acids formed in your discharge apparatus could have been made by bacteria contaminating the glassware or solutions. How did you respond to this concern?
Someone at a seminar had needled him that there were probably bacteria in there contaminating the solution. And he came back quite concerned, and told me that if I'd made a mistake, it would be better for me to retract than to be proven wrong by someone else. I knew that there were bacteria around, since the solutions were not handled under sterile conditions, but I also knew that they were not synthesizing the compounds. You could see some of the organic material actually being formed and dripping off the electrodes. I decided I was going to put an end to this doubt. I took the whole glass apparatus, filled it up with the gases, sealed it off, and put it in an autoclave for 18 hours instead of the usual 15 minutes required to kill bacteria. I then sparked the mixture and obtained the same results I had from the apparatus that had not been sterilized. No one has ever raised the issue of sterility since.

Did other labs rush to repeat your experiments after you published?
It's hard to say. One or two did, because they published papers saying they had obtained similar results. I suspect that others repeated it without reporting it. After a while, many experiments of this type (with variations) were done, and a substantial body of work has been accumulated.
In 1969, a meteorite fell in Murchison, Australia. Chemical analysis of the Murchison meteorite showed that it contained organic molecules. How does this discovery relate to your experiments on the origin of organic molecules?
True, what fell in Murchison, Australia (which is about 300 miles north of Melbourne), was a meteorite that contained organic material. Previously a number of these organic-containing meteorites had fallen and had been sitting in museums. They unquestionably contained organic material, but when they were analyzed for amino acids and other organic compounds, there were two problems. One was that while sitting in the museums they had been contaminated, and the other was that the analytical methods at the time were not adequate to detect and identify the small amounts of organic molecules. But by 1969, when the Murchison meteorite fell, the analytical methods had improved and the meteorite was fresh, so there weren't quite the contamination problems. So, when the meteorite was analyzed by a team at the NASA Ames Research Center, it turned out that amino acids were present at the parts per million level. These amino acids were very similar to the ones that had been made in the electrical discharge 15 years earlier. They did, however, find some additional amino acids that I hadn't obtained. So I repeated the electrical discharge experiments with a little modification, and I was able to show that all the amino acids in the Murchison meteorite were also obtained in the electrical discharge apparatus.
The significance of this is that we had been doing these prebiotic experiments not knowing whether this really took place, or if it was just a model experiment that bore no relation to reality. The similarity of the Murchison organic compounds and the electrical discharge compounds shows that this kind of synthesis took place on the parent body of the meteorite, probably an asteroid. This makes it plausible, but does not prove, that similar syntheses took place on the primitive Earth.
A few biologists have speculated that the first organic molecules on Earth arrived with extraterrestrial rocks such as the Murchison meteorite. What is your reaction to this idea?
Some organic substances certainly came in this way. There are problems with the survival of organic compounds on meteorites larger than a few meters in diameter, because they are not slowed up by the atmosphere before colliding with the Earth. In addition, sugars do not occur in Murchison, so some of the prebiotic synthesis must have occurred on the Earth. And I think that all but a few percent of the synthesis was done on the Earth.
Why doesn't such organic synthesis occur in the present world with our current atmosphere?
Organic synthesis of that sort doesn't take place now because of the presence of oxygen.
Each amino acid occurs in two three-dimensional forms: left- and right-handed versions that mirror each other. In your prebiotic synthesis experiments, both forms are produced, and yet only the left-handed molecules are common in life, especially in proteins. How do you account for this?
Well, that's been an outstanding question for a long time. There really is no good explanation for how this came about. All prebiotic syntheses give equal quantities of the right (D) and left (L) forms. In fact, you can tell whether or not you've got contamination if there's an excess of L-amino acids. That was the reason the amino acid analysis of the Murchison meteorite was convincing: there were equal quantities of the D and L forms. Without that, the presence of amino acids would have shouted "Contamination!" There are a number of rational explanations why living organisms have all L-amino acids. It has to do with selective advantage and the ease of synthesis from building blocks having similar geometry. Although we don't know how this came about, I'm convinced that it happened about the time of the origin of life or shortly thereafter.
But why don't we have both "left handed" and "right handed" species?
This is a common question I get asked. An all-D organism is as good as an all-L organism. If life arose only once, then it was by chance that it used L-amino acids, but it could, with equal probability, have used all D-amino acids. If life arose many times, there would have been both D and L organisms. In the course of time, one of these would have acquired a selective advantage and would have outgrown all the others.

When you were first proposing your experiments and doing your experiments on prebiotic organic synthesis, were you already thinking of this as a first stage in the origin of life?
Of course. We understood the implications of our discovery; we wouldn't have done the experiment as an exercise in chemical synthesis.
After your provocative paper appeared in the journal Science, was there much reaction in the press?
Oh, yes. There was a deluge of requests for interviews and pictures and the like. I was really quite surprised. Time magazine had a whole page on the experiment. There was a Gallup poll taken on whether or not people felt life could ever be made in a test tube. The opinion was largely no.
Assuming that mechanisms similar to those at work in your laboratory simulations produced organic molecules on the early Earth, what were some subsequent developments that were important to the origin of life?
The essential process is that of self-replication. Thus we need to find the prebiotic polymers that can contain genetic information. These may be RNA-like molecules, but they could have been quite different.
How early do you think life began on Earth?
Nobody really knows. The Earth is 4.5 billion years old. And the earliest evidence for life is about 3.5 billion. So there's a billion-year period in between. There have been various proposals about the temperature of early Earth: that it was cold when it formed or that it was molten. But prebiotic synthesis did not start until the Earth got down to a low enough temperature for the organic material to be stable: that is, below about a hundred degrees. We're not sure when that happened, but we assume maybe 4 billion years ago. That still leaves hundreds of millions of years in between the origin of suitable conditions on the Earth and the oldest known fossils, and we just don't know when life began during the period.
In what sort of place do you think life began on Earth?
The usual assumption is that it began in the ocean. But you can legitimately propose that some of the processes occurred in different areas. For example, some of the polymerization reactions that made larger organic molecules probably occurred on beaches that had dried out and heated up, and some may have occurred in hot springs. But the oceans form the bulk of the area where organic reactions could take place, and I think most of the chemistry took place there.
One hypothesis is that the first organisms arose in the hot water around volcanic vents on the sea floor. You and a colleague, Jeffrey Bada, have challenged this view in a recent publication. Would you describe your experiments?
The proposal was that life originated in these submarine vents. Water flows out of these vents at 350°C. This water is seawater that flows through the deep-sea sediments and is heated by molten rock. The water comes out in a matter of minutes through these black smoker chimneys, and then cools. Living around these vents are some very interesting creatures. There are tubeworms, clams, and the like. These organisms are not living at 350°C, but at around 37°C. And they live off the metabolic products of bacteria in a sort of symbiotic relationship.
The hypothesis is that water and gases start out at 350°C; the amino acids are made, polymerized in the vents; and then somehow the proteins and other polymers are organized into living organisms by the time they get out of the vent. The problem with this hypothesis is that organic compounds cannot be synthesized at 350°C. They would be destroyed. We did an experiment to illustrate this by taking some amino acids and heating them up in a test tube to 350°C. They decomposed before we even got them to that temperature. Furthermore, you can make polymers by heating amino acids, but you have to heat them dry. Well, in a submarine vent, it's obviously not dry. Even if you get these polymers of amino acids, that is not a living organism by any means: it has no genetic material. So on three or four counts the hypothesis is invalid.
If prebiotic synthesis of organic compounds led to the origin of life here on Earth, do you think this is a common process elsewhere in the universe?
I feel it is. But the problem is that we don't know that much about the subsequent steps. Making organic molecules such as amino acids, pyrimidines, purines, and sugars is easy. But how to organize them into the first living organism has not been worked out. So it's very difficult to estimate how frequently this might have occurred. My feeling, though, is that it is very frequent, and that the Earth is not unique in having life.
What work is presently going on in your laboratory?
I'm still studying the synthesis of prebiotic organic compounds, using atmospheres other than methane. There is considerable controversy about the composition of the primitive atmosphere. One of the problems is that methane and ammonia are decomposed by ultraviolet light rapidly. So the question is, could we have had that kind of atmosphere? And if the atmosphere was different, did it still lend itself to organic synthesis?
We have been doing experiments using carbon monoxide and carbon dioxide. You can make organic material with these kinds of atmospheres, but only if there is molecular hydrogen around. It's not that easy to get molecular hydrogen in large amounts into the atmosphere because it tends to escape from the atmosphere into outer space. Another problem that we have been working on in the laboratory is to try and figure out what primitive RNA was like. RNA is thought to have preceded DNA as the genetic material, but there was some precursor polymer to RNA.
Speaking of DNA, it just occurred to me that your famous paper was published the same year that Watson and Crick published their celebrated paper on the double helix-the structure of DNA, the genetic material.
Yes, their paper was in the April 25 issue of Nature and mine was in the May 15 issue of Science. I should mention that until then, most biologists thought that the genetic material was protein rather than DNA. That is why the prebiotic synthesis of amino acids was so striking.
Finally, what advice can you offer to students who are just beginning their college science education and are thinking about a career in research?
I think freshmen and sophomores should get a good background in chemistry and biology. Chemistry is very important because if you don't learn it as an undergraduate, you're probably not going to learn it very well as a graduate student. By the time they're juniors or seniors, students should probably do a little project in a research lab. Since biology is going more and more in a molecular direction, a strong background in chemistry and the ability to be efficient in the lab are very important.
©2005 Pearson Education, Inc., publishing as Benjamin Cummings