
To determine how much hydrogen peroxide (substrate) has been broken down by catalase at varying times, you measure the amount of peroxide remaining in each flask.
|
|

|
To determine how much hydrogen peroxide (substrate) has been broken down by catalase at varying times, you measure the amount of peroxide remaining in each flask. |
|
|
|
|
|
|
|
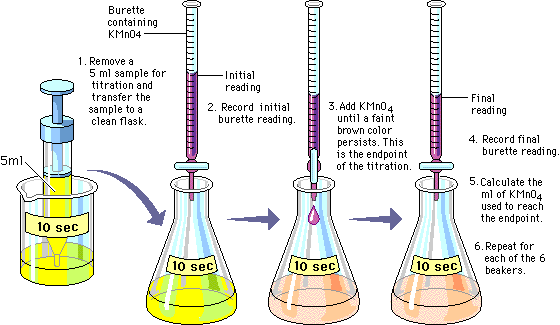
You slowly add KMnO4, which is purple, to the flask. The peroxide in the flask causes the KMnO4 to lose color when the solution is mixed thoroughly. When all the peroxide has reacted with KMnO4, any additional KMnO4 will remain light brown or pinkish even after you swirl the mixture. This is the endpoint. Record the amount of KMnO4 you have used. (The more KMnO4 you use, the more peroxide is in the flask.) |
|
|
|
|