
Recall that a hydrogen bond is a weak interaction between a hydrogen atom of one molecule and, in this case, the oxygen of another molecule.
Water is a polar molecule, with the region around the oxygen atom having a slight negative charge and the regions around the hydrogen atoms having a slight positive charge. In water, the negative regions on one molecule are attracted to the positive regions on another, and the molecules form hydrogen bonds.
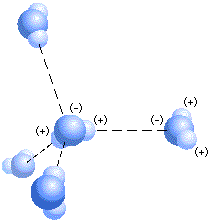
Notice in the figure above that each water molecule can form four hydrogen bonds. In liquid water, the average lifetime of a hydrogen bond is very short. However, the cumulative effect of hydrogen bonding among molecules makes liquid water much more cohesive than other liquids.